I joined the Hammer Lab as a postdoctoral research fellow in September 2014. Previously, I completed my graduate training at the University of Utah, where I studied pathways that regulate normal mammary gland development and how they may be disrupted during breast cancer progression. In the Hammer Lab, I am studying newly identified Wnt pathway target genes and how they contribute to adrenal homeostasis and cancer.
See More
The adrenal gland is one of the most common sites for neoplasia in humans, affecting an estimated 4-7% of the population1. While most adrenal tumors are benign adenomas discovered incidentally1, highly aggressive adrenal malignancies also form. In particular, adrenocortical carcinoma (ACC), which arises in the adrenal cortex, is often diagnosed late and progresses rapidly, resulting in significant morbidity and poor outcomes. Although ACC is a rare disease 2,3, current therapies have shown limited potential. Consequently, more effective therapies are critically needed for the treatment of ACC.
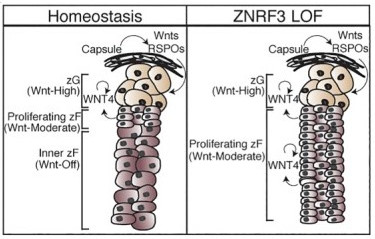
To develop more targeted therapeutic strategies, large-scale genomic studies, including The Cancer Genome Atlas Project, have characterized the genetic landscape of ACC4-6. In addition to known driver genes, these studies identified recurrent alterations in genes not previously associated with ACC. Specifically, homozygous deletion or loss of function mutation in the cell-surface transmembrane E3 ubiquitin ligase zinc and ring finger 3 (ZNRF3) was found in nearly 20% of human ACC samples. Interestingly, ZNRF3 is a known β-catenin target gene shown to negatively regulate Wnt signaling in other tissues7,8. As our lab and others have previously demonstrated an essential role for Wnt signaling in normal adrenal gland formation and homeostasis, we hypothesized that ZNRF3 acts in the adrenal cortex to regulate progenitor function.
To examine the role of ZNRF3 in adrenal biology, we generated a conditional knockout (cKO) mouse model and observed marked adrenal hyperplasia in the absence of ZNRF39. This substantial increase in adrenal size was accompanied by histological loss of normal adrenal architecture, primarily due to proliferative expansion of the inner, differentiated cortex. Moreover, using high-resolution single-molecule approaches, we discovered a Wnt signaling gradient in the normal adrenal cortex that is disrupted upon loss of ZNRF3. Unlike β-catenin gain-of-function models, which induce high Wnt activation and expansion of the peripheral cortex, ZNRF3 loss triggered activation of moderate-level Wnt signaling that resulted in proliferative expansion only of the histologically and functionally distinct inner cortex. To test the functional significance of these results, we genetically reduced the dosage of β-catenin, which significantly reversed the ZNRF3-deficient phenotype. Thus, our results demonstrate a critical role for ZNRF3 in homeostatic maintenance of the adrenal cortex through spatiotemporal control of Wnt signaling. Moving forward, our studies are focused on defining the long-term consequences of ZNRF3 loss in the adrenal cortex and using this model to test new potential therapeutic strategies for the treatment of adrenal cancer.
References
My name is Typhanie Dumontet, I joined Dr. Hammer's lab at the University of Michigan in 2018 as a post-doctoral research fellow, where I currently combine single-cell technology and newly developed mouse models to uncover transcriptional programs participating in adrenal differentiation.
See More
I obtained a Master's Degree and a Ph.D. in Genetics and Physiology from Clermont-Auvergne University (France) in 2017.
In Dr. Martinez's lab, my thesis work aimed at investigating the cellular origin of adrenal hyperplasia caused by mutations in a PKA-related gene using genetic approaches of cell lineage in mouse specie (Dumontet et al., 2018_JCI Insight). I also performed in vitro and in vivo studies to understand the effects of PKA activation on global protein SUMOylation (Dumontet et al., 2019_FASEB). During my Ph.D. training, I became highly interested in the mechanisms that govern cell differentiation.
In 2018, I joined Dr. Hammer's lab at the University of Michigan as a post-doctoral research fellow, where I currently combine single-cell technology and newly developed mouse models to uncover transcriptional programs participating in adrenal differentiation. More broadly, I am particularly interested in understanding how the deregulation of these programs might contribute to pathologies in humans.
With Dr. Hammer as a mentor, my long-term goal is to develop the necessary training, skills, and experience to become an independent investigator by implementing new technology and mouse models to address pathologic and therapeutic challenges of adrenal diseases
My research focuses on defining transcriptional programs that control adrenocortical development, determining mechanisms by which gene expression is regulated in adrenocortical cells, and exploring molecular pathways that can be targeted for the treatment of adrenal diseases.
See More
I am an Assistant Research Scientist in the laboratory of Dr. Gary Hammer at the University of Michigan. I received my Ph.D. in Neuroscience in the laboratory of Dr. Nira Ben-Jonathan at the University of Cincinnati, and completed my Postdoctoral training with Dr. Jessica Schwartz at the University of Michigan. I was appointed a research faculty member in the Department of Internal Medicine and the division of Metabolism, Endocrinology, and Diabetes (MEND) at the University of Michigan in 2013.
My research focuses on defining transcriptional programs that control adrenocortical development, determining mechanisms by which gene expression is regulated in adrenocortical cells, and exploring molecular pathways that can be targeted for the treatment of adrenal diseases.
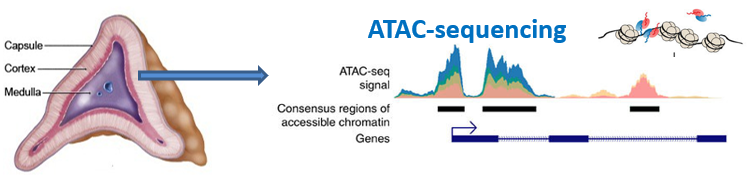
The orphan nuclear receptor SF-1, is an important mediator of normal growth and homeostasis in the adrenal gland. This transcription factor regulates steroidogenesis in differentiated adrenal tissue but also is essential for adrenal development. Adrenal glands and gonads do not develop in SF-1 deficient mice, highlighting the importance of SF-1 expression during development. While the enhancer that drives SF-1 expression in the fetal adrenal has been characterized, the location of the regulatory element for SF-1 in adult tissue remains unknown. One of my research projects is aimed at determining the genomic location of the adult SF-1 enhancer by assessing chromatin accessibility using ATAC-Seq during development. Combining ATAC-Seq with ChIP-Seq and RNA-Seq is a novel approach for studying the relationship between genome structure, chromatin modifiers and changes in global patterns of gene expression. We are using this integrative approach to define transcriptional mechanisms by which endocrine (ACTH) and paracrine (Wnt) factors coordinate dynamic regulation of steroidogenic genes expressed in adrenocortical cells.
In addition to exploring mechanisms that regulate transcriptional activity in the adrenal cortex, I am interested in exploring molecular pathways that can be targeted for the treatment of adrenal diseases, specifically Adrenocortical cancer (ACC). Adrenocortical cancer (ACC) is a rare cancer of the adrenal cortex with limited treatment options. There exists an unmet need for new drugs or drug combinations that target key regulatory pathways in ACC cells, with the goal of decreasing tumor growth and, ultimately, increasing survival of patients with ACC. My recent studies of ATR-101, a novel oral ACC drug candidate currently in development, identified the mechanism by which this drug induces death in an ACC cell line and in the adrenal cortex of dogs. I am currently working on follow-up studies aimed at assessing synergistic effects between ATR-101 and other chemotherapeutic compounds for the treatment of ACC.
Adrenocortical carcinoma (ACC) is a deadly disease with few therapies. We recently led the NCI-sponsored The Cancer Genome Atlas study on ACC (ACC-TCGA), in which we performed a multiplatform high-resolution molecular characterization of ACC. My work is focused on translating insights from this comprehensive study to develop new prognostic tools, identify targeted therapies, and deepen the molecular understanding of ACC and adrenocortical biology. Recent projects I am currently leading are focused on the following areas: 1.) molecular biomarker development, 2.) tumor epigenetics and 3.) steroid hormone/immune cell crosstalk.
See More
The adrenal glands are paired endocrine organs that produce steroid hormones and catecholamines critical for life. Adrenocortical carcinoma (ACC) is a deadly cancer of these glands for which few therapies are available. We recently led the multi-institutional NCI-sponsored The Cancer Genome Atlas (TCGA) study on ACC (ACC-TCGA) to comprehensively characterize the molecular landscape of these tumors using multiomics approaches (Zheng, Cancer Cell, 2016). While illustrative, multi-omics technologies are not routinely available for clinical applications; therefore, the ultimate goal of our current research is to translate molecular findings of ACC-TCGA to simplified tools and therapeutic strategies to ultimately improve patient care.
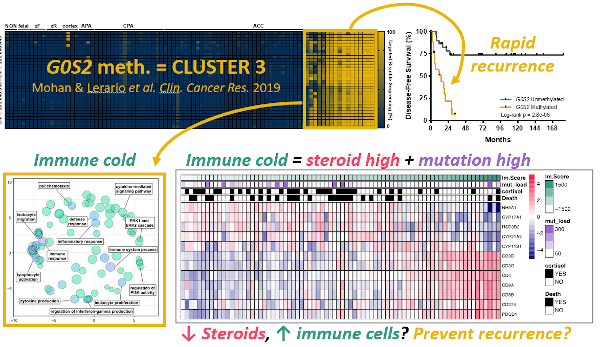
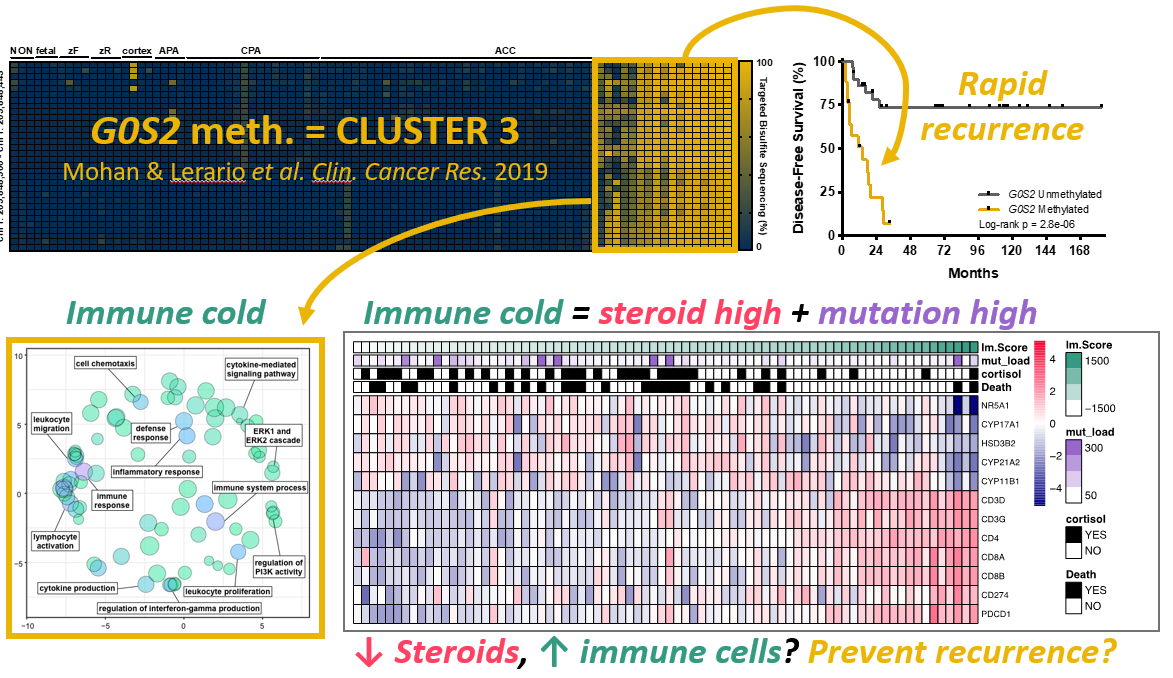
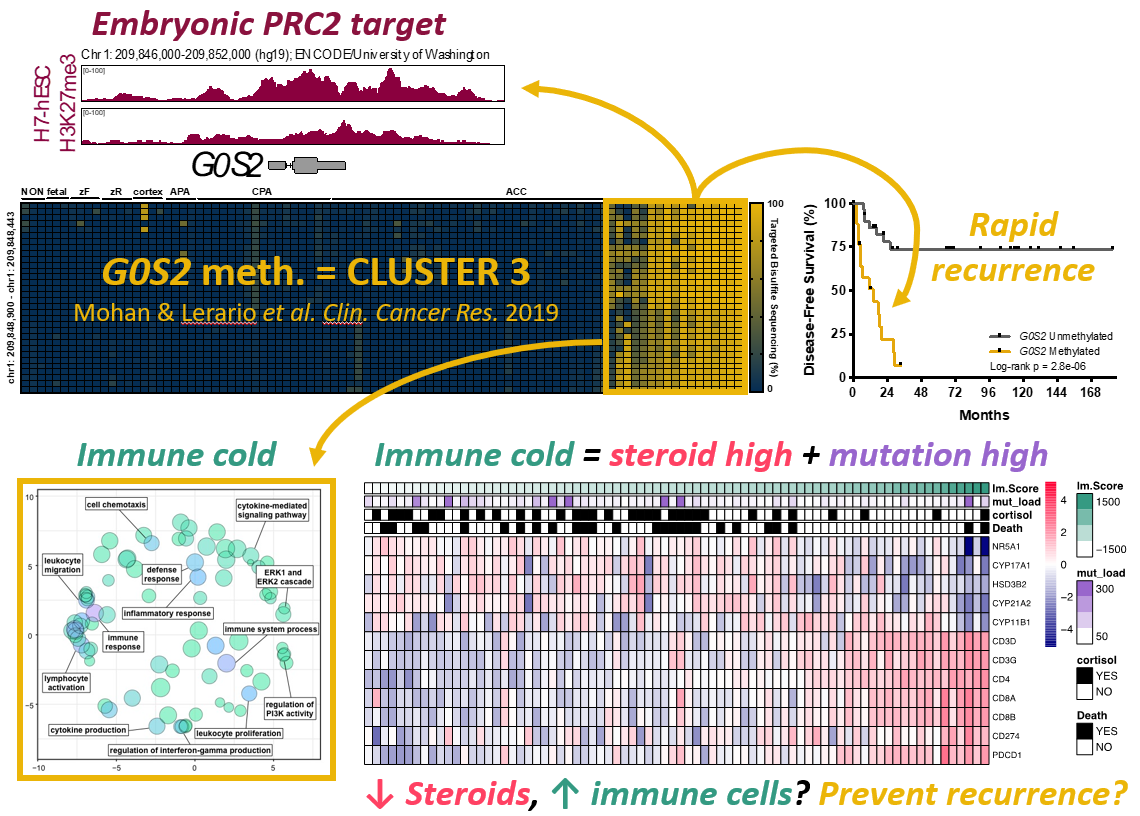
1.) Molecular biomarker development - One of the major findings of ACC-TCGA was that ACC comprises three distinct, equally prevalent molecular subtypes, here designated cluster 1, cluster 2, and cluster 3. These subtypes exhibit strikingly different prognosis; notably, patients with cluster 3 tumors account for ~70% of recurrences and >50% of deaths. Because cluster 3 ACC bear such dismal outcomes, we are focusing our efforts on developing tools to prospectively identify this aggressive subtype as soon as a patient receives a diagnosis of ACC, so we may ultimately exploit unique vulnerabilities that might lead to improved and efficient therapies. A distinguishing feature of cluster 3 ACC is a profound disruption in epigenetic patterning, leading to widespread CpG island hypermethylation (CIMP-high). We have recently developed a simplified, PCR-based molecular assay to unambiguously identify CIMP-high ACC by measuring the methylation level of the G0S2 locus (Mohan & Lerario, Clinical Cancer Research, 2019). We are now using this biomarker in prospective clinical studies and international clinical trials to interrogate whether it predicts response to specific adjuvant therapies, through work supported by the US Department of Defense.
2.) Tumor epigenetics - Given the striking association between abnormal epigenetic patterning and cluster 3 ACC, we sought to explore the role of epigenetics in the molecular pathogenesis of these tumors. We identified that CpG island hypermethylation is directed to embryonic targets of the Polycomb repressive complex 2 (PRC2), a complex of proteins that silence gene expression by writing the repressive histone mark H3K27me3. Interestingly, the histone methyltransferase member of PRC2, EZH2, is highly expressed and catalytically active in CIMP-high ACC, suggesting crosstalk between DNA methylation and histone methylation in the molecular pathogenesis of this aggressive molecular subtype. Since EZH2 activity is pharmacologically targetable using FDA-approved agents, we are currently studying the molecular consequences of perturbing the PRC2 function in an in vitro model of CIMP-high ACC and in vivo model of adrenal regeneration.
3.) Steroid hormone/immune cell crosstalk - We have demonstrated that high steroidogenic activity and low immune infiltration are also distinguishing features of cluster 3, despite that these tumors bear the highest mutational burden across ACC (Zheng, Cancer Cell, 2016; Mohan & Lerario, Clinical Cancer Research, 2019). It is well known that steroid hormones like glucocorticoids are immune suppressive, so much so that these agents are used to suppress the immune system in patients receiving organ transplants. We therefore hypothesize that increased intra-tumoral steroid production by cluster 3 ACC excludes immune cells despite the high mutational burden. We are currently investigating whether targeting steroid production or nuclear receptor functions might enhance the anti-cancer immune response to ACC, and therefore, improve the effects of recently FDA-approved immunotherapy agents through work also supported by the US Department of Defense.
References:
Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM,Else T, Knijnenburg TA, Ciriello G, Kim S, Assie G, Morozova O, Akbani R, Shih J,Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ,Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ,Fragoso MCBV, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE,Asa SL, Bertherat J, Fassnacht M, Wheeler DA; Cancer Genome Atlas ResearchNetwork, Hammer GD, Giordano TJ, Verhaak RGW. Comprehensive Pan-GenomicCharacterization of Adrenocortical Carcinoma. Cancer Cell. 2016 May9;29(5):723-736. doi: 10.1016/j.ccell.2016.04.002. Erratum in: Cancer Cell. 2016 Aug 8;30(2):363. PubMed PMID: 27165744; PubMed Central PMCID: PMC4864952
Mohan DR, Lerario AM, Else T, Mukherjee B, Almeida MQ, Vinco M, Rege J,Mariani BMP, Zerbini MCN, Mendonca BB, Latronico AC, Marie SKN, Rainey WE,Giordano TJ, Fragoso MCBV, Hammer GD. Targeted Assessment of G0S2 MethylationIdentifies a Rapidly Recurrent, Routinely Fatal Molecular Subtype ofAdrenocortical Carcinoma. Clin Cancer Res. 2019 Jun 1;25(11):3276-3288. doi:10.1158/1078-0432.CCR-18-2693. Epub 2019 Feb 15. PubMed PMID: 30770352.
My project focuses on defining the role of adrenal capsule-derived WNT2B. I have generated a Wnt2b knockout mouse model and am using various in vitro techniques to understand the role of WNT2B in mediating adrenocortical progenitor cell fate and zG identity, a crucial process in normal adrenal development and homeostasis.
See More
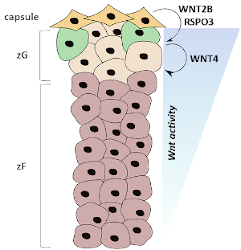
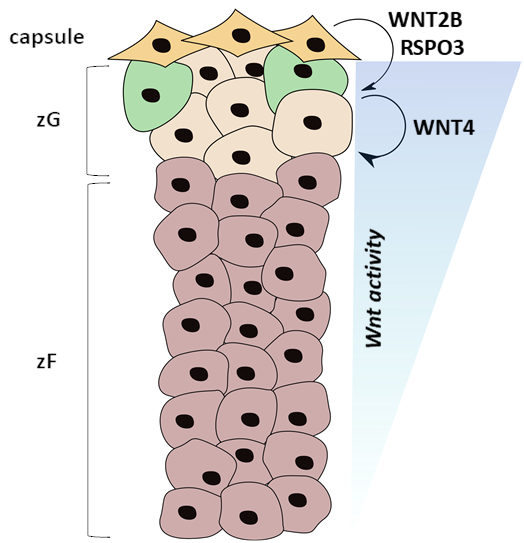 Wnt signaling is an essential pathway in both the development of several tissues and tissue homeostasis throughout life and is frequently altered in adrenal cancer. The Wnt pathway is especially important for maintaining various tissue-specific stem/progenitor cell pools, and adrenocortical progenitor cells are not unique—they are dependent upon Wnt signaling for maintenance, as various data show that constitutive or inactive Wnt signaling leads to adrenocortical hyperplasia or late-stage adrenal failure, respectively. Until recently, only WNT4 has been studied in the adrenal as it is highly expressed in the zG and maintains aldosterone production. However, we recently found Wnt2b to be expressed exclusively in the overlying adrenal capsule alongside RSPO3, a Wnt signaling potentiator that is necessary for proper adrenal zonation and zG identity. Therefore, we hypothesize that WNT2B is the main activator of adrenocortical Wnt signaling that maintains both the progenitor cell pool and the identity of the overall zG in which the progenitor cells reside. Interestingly, data from my work implicate a role for WNT2B not only in the undifferentiated state of progenitor cells, but also in expression of zG-specific steroidogenic genes. These results suggest that WNT2B may have a unique effect on both progenitor and steroidogenic cells of the adrenal zG. Future work will seek to define these unique roles and the transcriptional programs activated by WNT2B in these two cell populations to further understand how Wnt signaling is hijacked to initiate or promote adrenal cancer.
Wnt signaling is an essential pathway in both the development of several tissues and tissue homeostasis throughout life and is frequently altered in adrenal cancer. The Wnt pathway is especially important for maintaining various tissue-specific stem/progenitor cell pools, and adrenocortical progenitor cells are not unique—they are dependent upon Wnt signaling for maintenance, as various data show that constitutive or inactive Wnt signaling leads to adrenocortical hyperplasia or late-stage adrenal failure, respectively. Until recently, only WNT4 has been studied in the adrenal as it is highly expressed in the zG and maintains aldosterone production. However, we recently found Wnt2b to be expressed exclusively in the overlying adrenal capsule alongside RSPO3, a Wnt signaling potentiator that is necessary for proper adrenal zonation and zG identity. Therefore, we hypothesize that WNT2B is the main activator of adrenocortical Wnt signaling that maintains both the progenitor cell pool and the identity of the overall zG in which the progenitor cells reside. Interestingly, data from my work implicate a role for WNT2B not only in the undifferentiated state of progenitor cells, but also in expression of zG-specific steroidogenic genes. These results suggest that WNT2B may have a unique effect on both progenitor and steroidogenic cells of the adrenal zG. Future work will seek to define these unique roles and the transcriptional programs activated by WNT2B in these two cell populations to further understand how Wnt signaling is hijacked to initiate or promote adrenal cancer.
Adrenocortical carcinoma (ACC) is a rare cancer of the adrenal gland, and to date the only therapy with potential to cure is surgery. ~75% of patients develop deadly metastatic disease, highlighting an urgent need for improved medical agents. In The Cancer Genome Atlas study on ACC, we identified a rapidly recurrent, routinely fatal molecular subtype of ACC characterized by tumor CpG island hypermethylation and a unique transcriptional program and somatic alteration profile. My work is focused on developing molecular tools to enable accurate prospective identification of patients with this ACC subtype, and on refining the understanding of dysregulated epigenetic programs.
See More
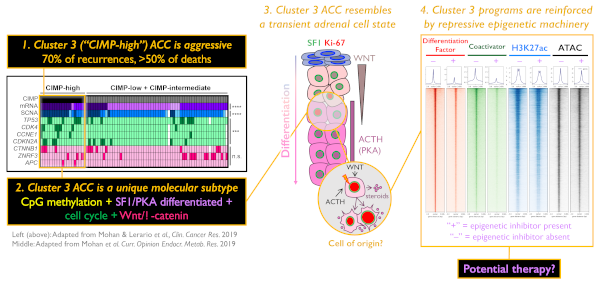
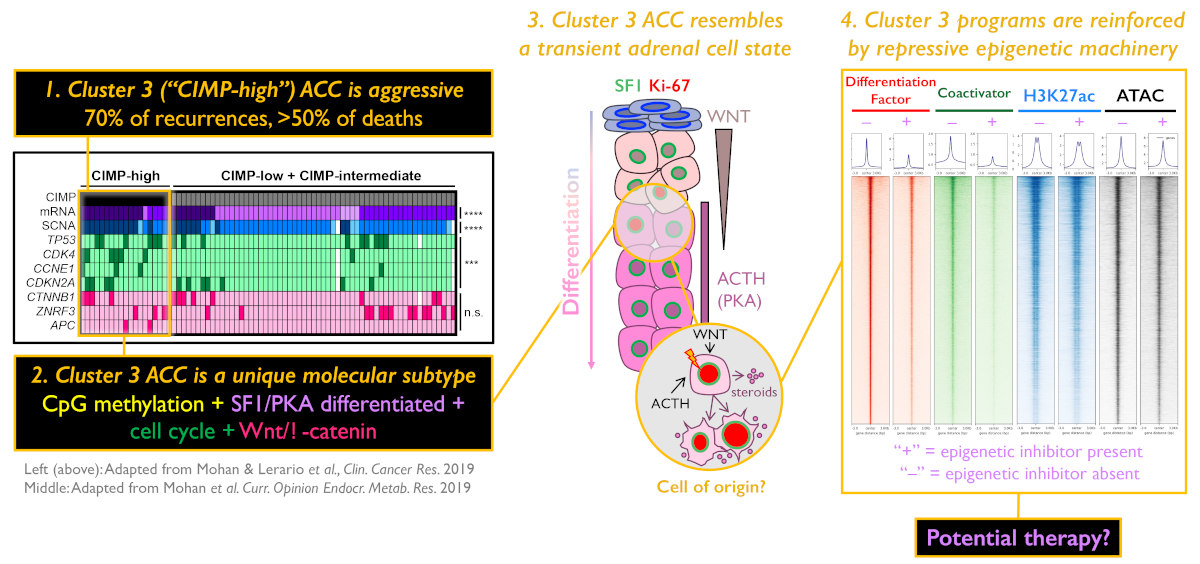
The adrenal glands are paired endocrine organs that reside above each kidney and produce steroids and catecholamines critical for life. Adrenocortical carcinoma (ACC) is a rare, poorly understood cancer of these glands with few therapies. We recently identified that patients with tumor CpG island hypermethylation ("CIMP-high") have rapidly recurrent, routinely fatal disease, accounting for 70% of recurrences and > 50% of deaths (The Cancer Genome Atlas study on ACC [ACC-TCGA], Zheng et al., Cancer Cell 2016; Mohan & Lerario et al. Clinical Cancer Research 2019). Recently, we developed a single-gene DNA methylation-based biomarker to capture these tumors and demonstrated that this methylation signature is actually a surrogate for a molecular subtype of ACC ("Cluster 3"), characterized by sustained Wnt/β-catenin signaling, SF1/PKA-driven steroidogenic differentiation, and cell cycle activation (Mohan & Lerario et al. Clinical Cancer Research 2019). With the goal of ultimately developing a targeted therapy to combat Cluster 3 ACC, we have now focused our studies on elucidating the aberrant epigenetic and transcriptional landscape that defines these tumors.
Our subsequent analyses of ACC-TCGA data have revealed that Cluster 3/CIMP-high tumors are characterized by DNA methylation deposited in regions of the genome that are targets of the Polycomb repressive complex 2 (PRC2) in embryonic stem cells. The physiological role of PRC2 is to safeguard stem cell pluripotency. It accomplishes this through its catalytically active member, EZH2, which deposits histone H3 lysine 27 trimethylation (H3K27me3) in promoter CpG islands free of DNA methylation. Despite this well characterized function, both gain and loss of EZH2/PRC2 activity prevail in a variety of human cancers, suggesting both oncogenic and tumor suppressive roles. In some cancers EZH2 acts as a transcriptional co-activator, in other cancers loss of PRC2 promotes expansion of an undifferentiated cell population, and some models even propose that catalytically active PRC2 directs DNA methylation in cancer.
We have since identified that despite PRC2 target DNA methylation, EZH2 expression is high and coupled to H3K27me3 in Cluster 3 ACC. Using IP-MS, ChIP-seq, ATAC-seq and methylation array profiling in an in vitro model of Cluster 3 ACC, we have demonstrated that canonical PRC2 is fully assembled, depositing H3K27me3 at many inaccessible regions of the genome, but is excluded from sites that are targeted for aberrant DNA methylation. Intriguingly, our data has revealed that EZH2 interacts with a transcriptional co-activator that is known to drive aberrant transcriptional programming in Cluster 3 tumors. Pharmacological inhibition of EZH2 activity reverses the Wnt/β-catenin signaling, SF1/PKA-driven steroidogenic differentiation, and cell cycle activation that define Cluster 3 (measured by RNA-seq), and our recent ChIP-seq analysis suggests that this is through a requirement for catalytically active EZH2/PRC2 to reinforce these programs. Our ongoing studies are focused on extending these observations to in vivo models of adrenocortical regeneration and evaluating a potential therapeutic role for EZH2/PRC2 inhibition in ACC.
I joined Hammer Lab in the Summer of 2020 to pursue my PhD degree as a student in the Department of Cell & Developmental Biology. My research focuses on adrenal development and homeostasis.
See More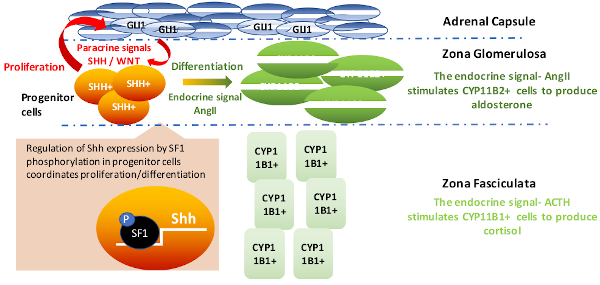
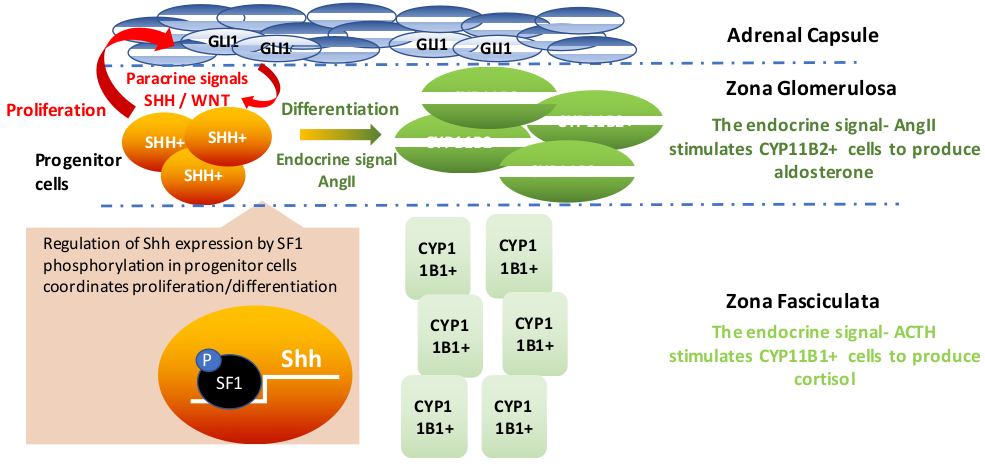
Steroidogenic factor 1 (SF1) is a key regulator in adrenal development and steroidogenesis. Our team has employed newly developed mouse models to show SF1 phosphorylation status is involved in regulating progenitor fate in the adrenal cortex. I am exploring how SF1 phosphorylation is linked to cell cycle in Shh-expressing progenitors, as well as how it is influencing progenitor behavior under endocrine stress.
I am a PhD candidate through the Department of Cell & Developmental Biology here at Michigan. I switched into the Hammer lab in Fall 2020 to continue my PhD training. My research interests lie where evolutionary and developmental biology, genetics, and regeneration and disease intersect.
See More
Research in the Hammer lab has contributed greatly to the molecular understanding of adrenocortical carcinoma (ACC), an aggressive cancer with poor prognosis and grim outcomes following current standard therapeutic treatments. My project focuses on elucidating the signaling mechanisms between ACC tumor cells and T cells of the immune system, and how intra-tumoral steroid production in ACC may be harnessed to evade immune infiltration. The findings of this project may inform novel immunotherapy treatments to improve the outcome for ACC patients.