Holly Hung, Ph.D.
Endocrinology Postdoctoral Research Fellow
(Member of the Hammer Lab Since August 2014)

In January 2015, the Hammer lab became my research home following completion of my PhD training at the University of Wisconsin in Cellular and Molecular Pathology. I am interested in studying factors that affect clinical prognosis in the development and progression of adrenal tumors. Specifically, my project in the Hammer lab investigates the contribution of Zac1/Plagl1 to critical aspects of the adrenal progenitor cell biology, adrenal cortex maintenance, and cancer.
While there is a high incidence of adrenocortical tumors in the general population (3-10%), the frequency of malignancy is low (1-2 cases per million adults per year) [1]. Sadly, the overall prognosis for patients with adrenocortical carcinoma (ACC) is poor (5-year survival rate is <35%), however individual outcomes are highly heterogeneous – suggesting distinct oncogenic mechanisms are driving disease progression and outcome [2].
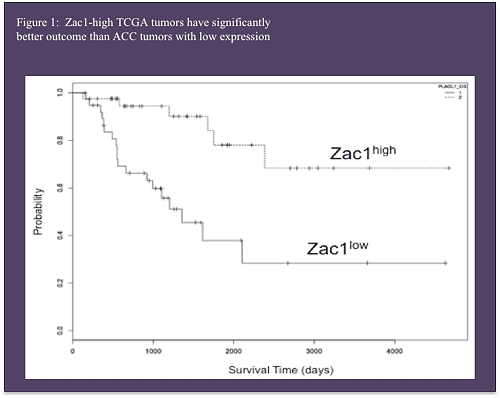
Advances in genomic analysis have generated new tools to distinguish molecular signatures of benign tumors and identify subtypes of malignant disease. The University of Michigan Endocrine Oncology Program has been utilizing these tools as the coordinating center for the NCI-sponsored adrenocortical carcinoma TCGA (The Cancer Genome Atlas) project to parse the genomic landscape of ACC. From the groundwork laid by the TCGA project, I am studying the oncogenic mechanisms that drive the best prognosis in ACC patients (Figure 1).
Molecular roles of ZAC1 in adrenal cortex development and cancer
Zac1, also known as Plag1 or Lot1 was initially identified in in a tissue culture model of ovarian cancer [3]. This gene encodes a C2H2 zinc finger protein thought to function as a tumor suppressor. With key developmental roles in many tissues including pituitary gland [4], retina [5], and skin [6], Zac1 has been shown to regulate cell cycle control and apoptosis in a cell-type specific manner. While it is know to be faintly expressed in adult adrenal glands, the normal function and biology of Zac1 is unknown. In cancer, Zac1/Plagl1 is a frequent target for gene silencing through DNA methylation. Importantly, inactivation of Zac1 expression in breast and ovarian cancer is thought to be a transformative step between benign and malignant tumor formation [7]. Our studies have shown Zac1 to be one of the most down regulated genes in pediatric ACC [8] and low Zac1 expression is part of the molecular signature of TCGA tumor groups with the worst clinical outcomes.
Despite the evident importance of Zac1 in adrenal tumorigenesis, the mechanism of action and gene targets of Zac1 in normal adrenal homeostasis and tumorigenesis are unknown. I hypothesize (1) Zac1/Plagl1 plays a role in limiting adrenal progenitor cell numbers during development, (2) tumors that harbor high Zac1 expression are derived from progenitor cell populations, and (3) downstream gene targets of Zac1 activity in adrenocortical tumors can drive the transformation of benign adrenocortical adenomas into malignant adrenocortical carcinoma (ACC).
To test these hypotheses I plan to track the lineage of Zac1 expressing cell populations using transgenic mouse reporters to determine Zac1 contribution to adrenal development and progenitor populations. To examine the tumor contributing potential of these cells, I am currently developing new tools to control Zac1 expression using lentiviral expression systems. I plan to use these systems to evaluate the effects of Zac1 loss and overexpression in normal adrenal cells and malignant ACC tumor cell in vitro and through ex vivo transplantation models in mice. The pursuit of downstream gene targets for Zac1 will be facilitated by in vivo ChIP-seq analysis to survey changes in Zac1 binding in normal adrenal homeostasis and cancer. Using these models I hope to determine the oncogenic mechanisms differentially controlled between Zac1 high and Zac1 low expressing tumors. Together the proposed experiments will provide insights into the developmental role of Zac1/Plagl1 and an improved understanding of tumorigenic transformation in adrenocortical carcinoma to aid in developing therapeutic interventions for ACC patients.
References
- Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD: Adrenocortical carcinoma. Endocr Rev 2014, 35:282-326.
- Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, Dousset B, Bertagna X, Bertherat J: Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab 2006, 91:2650-2655.
- Abdollahi A, Godwin AK, Miller PD, Getts LA, Schultz DC, Taguchi T, Testa JR, Hamilton TC: Identification of a gene containing zinc-finger motifs based on lost expression in malignantly transformed rat ovarian surface epithelial cells. Cancer Res 1997, 57:2029-2034.
- Pagotto U, Arzberger T, Theodoropoulou M, Grübler Y, Pantaloni C, Saeger W, Losa M, Journot L, Stalla GK, Spengler D: The expression of the antiproliferative gene ZAC is lost or highly reduced in nonfunctioning pituitary adenomas. Cancer Res 2000, 60:6794-6799.
- Ma L, Cantrup R, Varrault A, Colak D, Klenin N, Götz M, McFarlane S, Journot L, Schuurmans C: Zac1 functions through TGFbetaII to negatively regulate cell number in the developing retina. Neural Dev 2007, 2:11.
- Basyuk E, Coulon V, Le Digarcher A, Coisy-Quivy M, Moles JP, Gandarillas A, Journot L: The candidate tumor suppressor gene ZAC is involved in keratinocyte differentiation and its expression is lost in basal cell carcinomas. Mol Cancer Res 2005, 3:483-492.
- Abdollahi A, Pisarcik D, Roberts D, Weinstein J, Cairns P, Hamilton TC: LOT1 (PLAGL1/ZAC1), the candidate tumor suppressor gene at chromosome 6q24-25, is epigenetically regulated in cancer. J Biol Chem 2003, 278:6041-6049.
- West AN, Neale GA, Pounds S, Figueredo BC, Rodriguez Galindo C, Pianovski MA, Oliveira Filho AG, Malkin D, Lalli E, Ribeiro R, Zambetti GP: Gene expression profiling of childhood adrenocortical tumors. Cancer Res 2007, 67:600-608.





